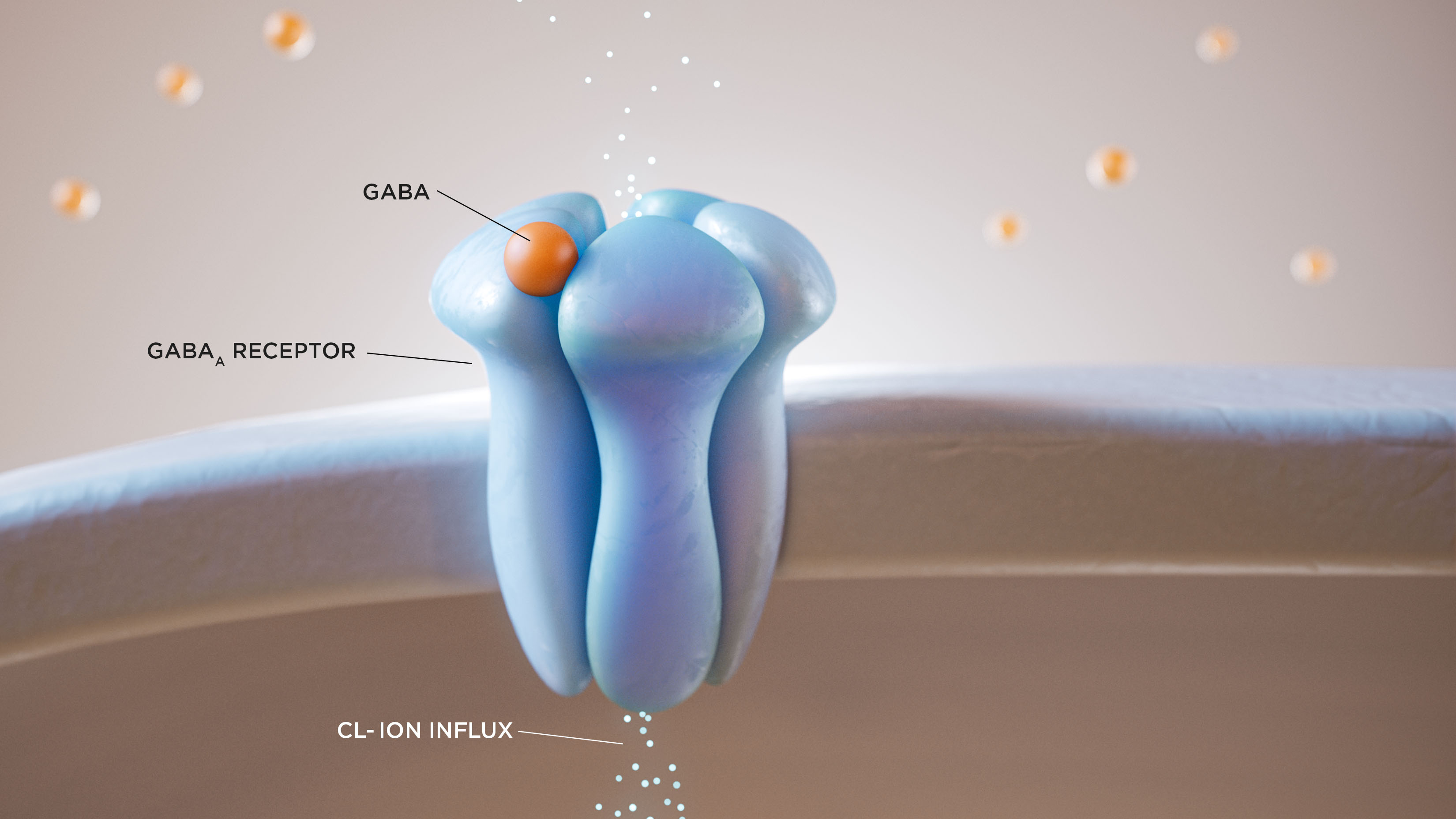
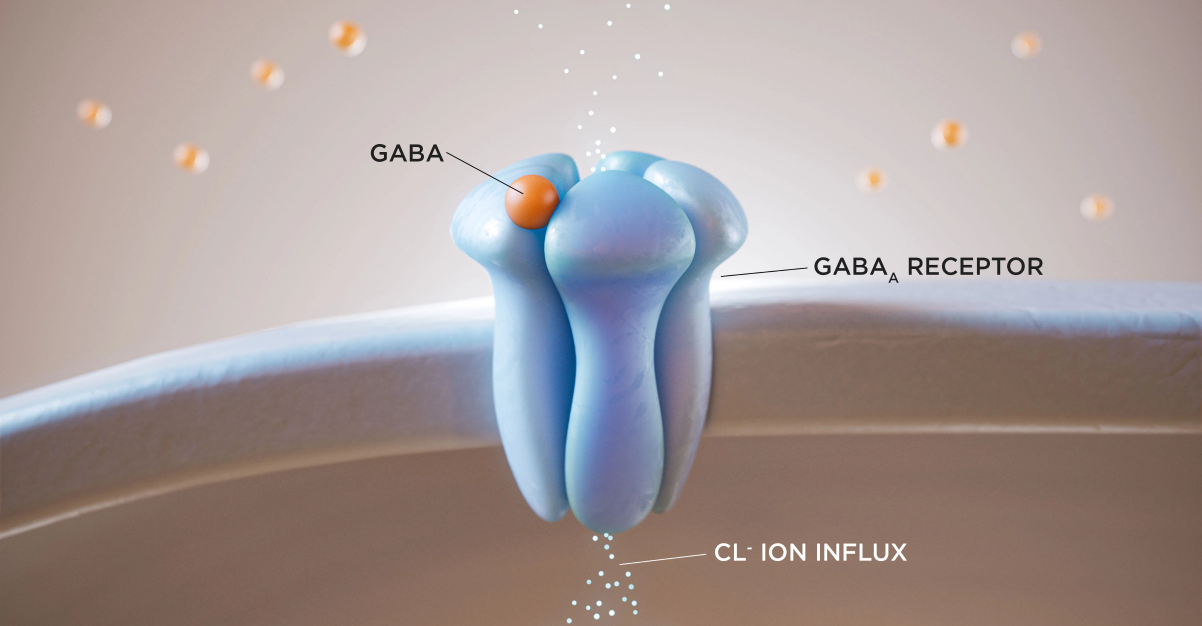
INDICATION AND USAGE
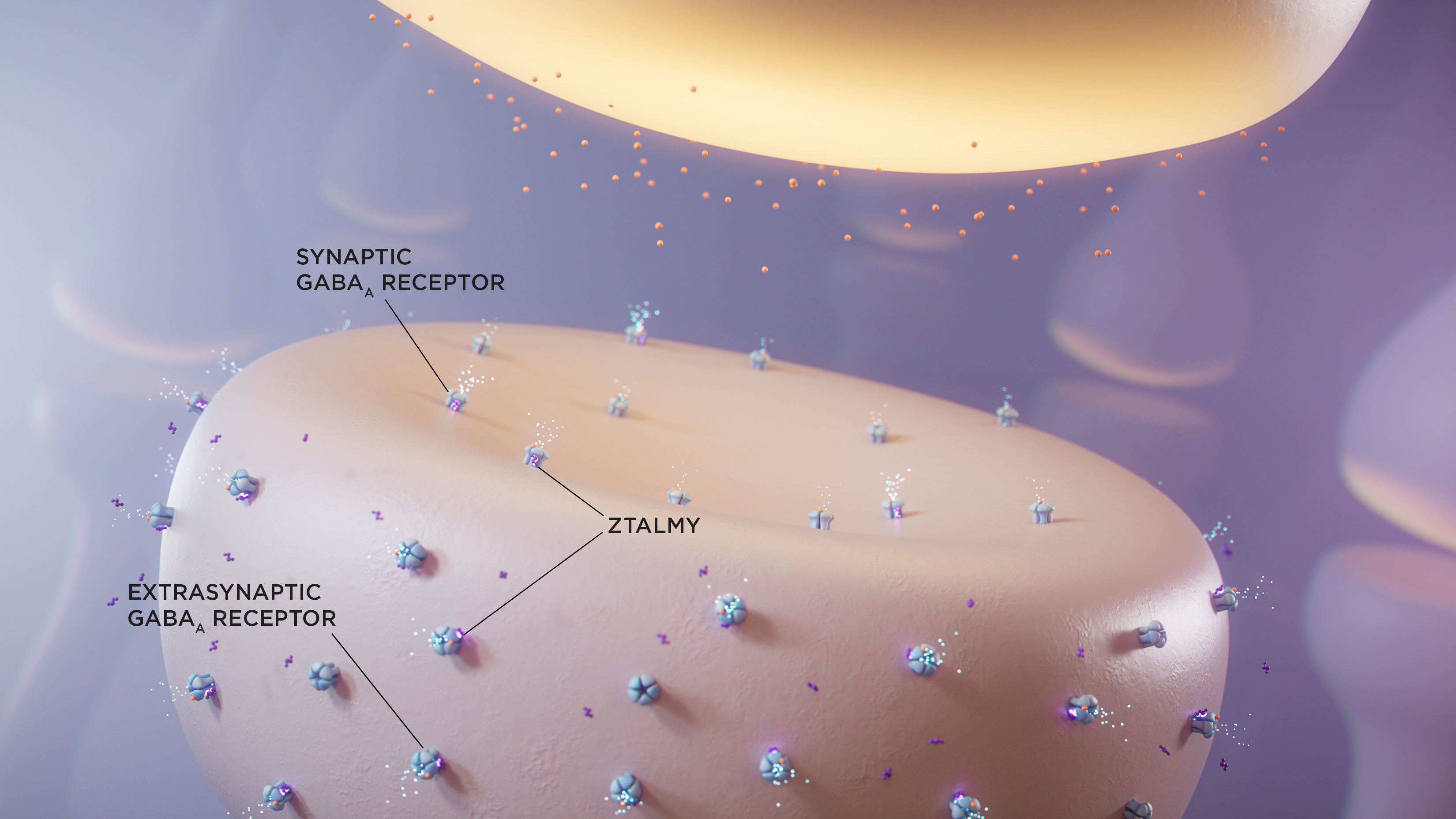
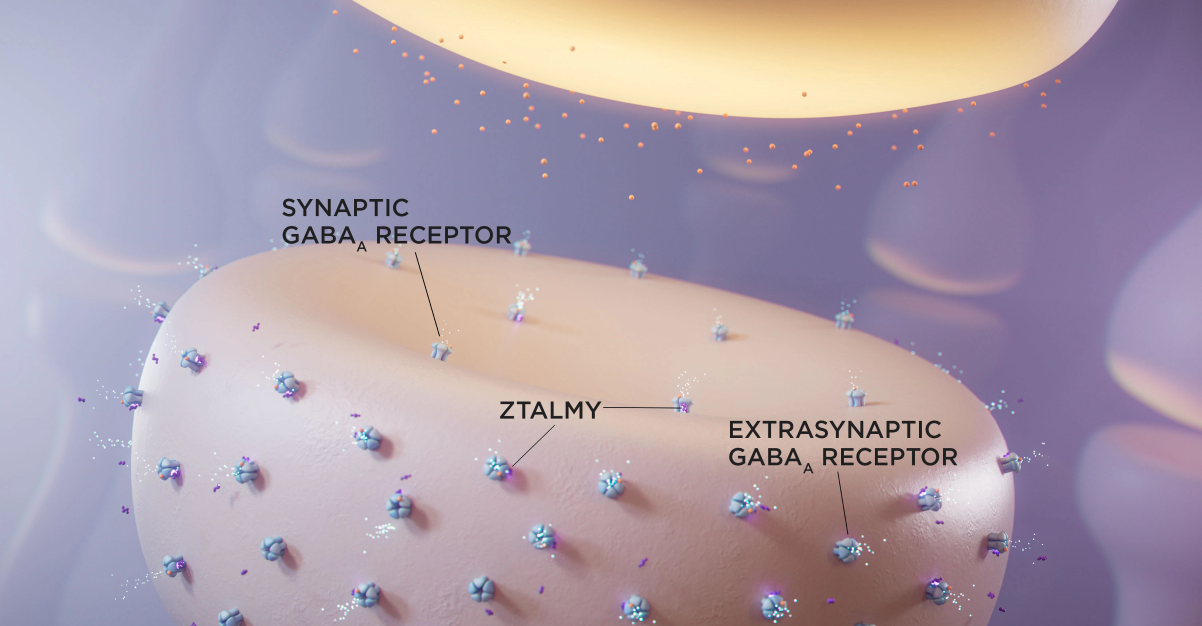
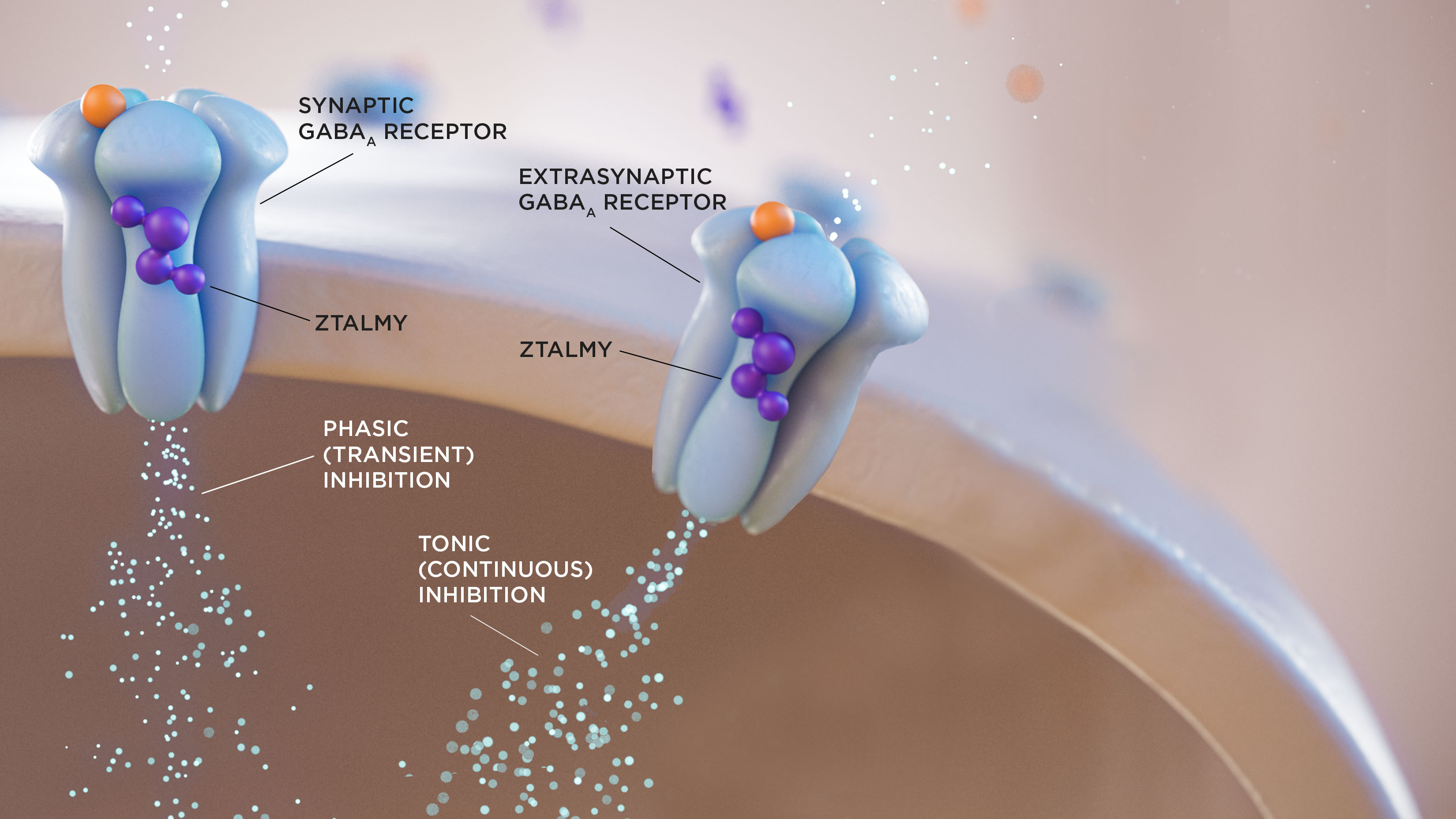
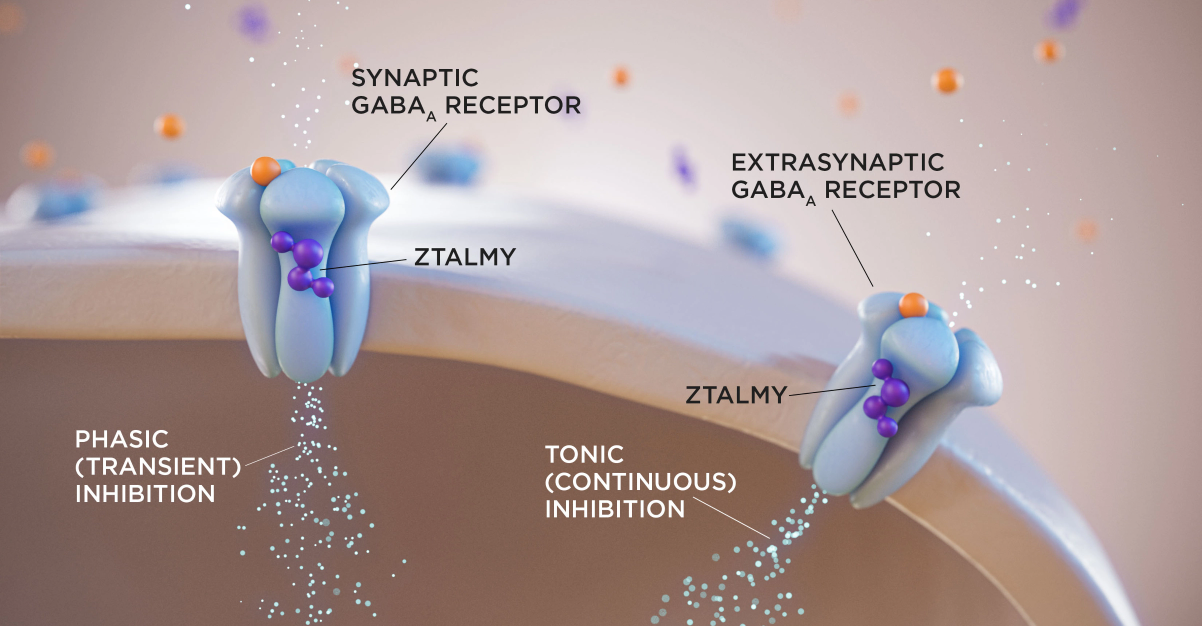
ZTALMY is indicated for the treatment of seizures associated with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD) in patients 2 years of age and older.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
-
•
Somnolence and Sedation:
ZTALMY can cause somnolence and sedation. In a clinical study somnolence and sedation appeared early during treatment and were generally dose related. Other CNS depressants, including opioids, antidepressants, and alcohol, could potentiate these effects. Monitor patients for these effects and advise them not to drive or operate machinery until they have gained sufficient experience on ZTALMY to gauge whether it adversely affects their ability to drive or operate machinery.
-
•
Suicidal Behavior and Ideation:
Antiepileptic drugs (AEDs), including ZTALMY, increase the risk of suicidal thoughts or behavior. Monitor patients taking ZTALMY for the emergence or worsening of depression, suicidal thoughts or behavior, or any unusual changes in mood or behavior. Advise patients, caregivers, and their families to be alert for these behavioral changes and report behaviors of concern immediately to healthcare providers. When considering ZTALMY, or any other AED, balance the risk of suicidal thoughts or behaviors with the risk of untreated illness. If these symptoms emerge during treatment, consider whether it may be related to the AED or the underlying illness.
-
•
Withdrawal of Antiepileptic Drugs:
As with most AEDs, withdraw ZTALMY gradually to minimize the risk of increased seizure frequency and status epilepticus. If withdrawal is needed because of a serious adverse event, rapid discontinuation can be considered.
ADVERSE REACTIONS
The most common adverse reactions (incidence of at least 5% and at least twice the rate of placebo) were somnolence (38%), pyrexia (18%), salivary hypersecretion (6%), and seasonal allergy (6%).
DRUG INTERACTIONS
Cytochrome P450 inducers will decrease ganaxolone exposure. Avoid concomitant use with strong or moderate CYP3A4 inducers; if unavoidable, consider a dosage increase of ZTALMY, but do not exceed the maximum recommended dosage.
USE IN SPECIFIC POPULATIONS
-
•
Pregnancy: Use caution when ZTALMY is administered to pregnant women as there are no adequate data on the developmental risk associated with use in pregnant women. In animal studies, developmental adverse effects were observed following exposure during organogenesis or throughout gestation and lactation.
-
•
Lactation: ZTALMY is excreted in human milk at concentrations resulting in a dose to the breastfed infant of less than 1% maternal dose. The effects of ZTALMY on milk production and the breastfed infant are unknown.
-
•
Hepatic Impairment: Administration of ZTALMY in patients with severe hepatic impairment (Child‑Pugh class C) results in elevated ganaxolone plasma concentrations. Therefore, dosage adjustment in these patients during titration and maintenance is required. No dosage adjustment is necessary in patients with mild (Child‑Pugh class A) or moderate (Child‑Pugh class B) hepatic impairment.
DRUG ABUSE AND DEPENDENCE
ZTALMY contains ganaxolone, a Schedule V controlled substance (CV). Advise patients of the potential for abuse and dependence. It is recommended that ZTALMY be tapered according to the dosage recommendations unless symptoms warrant immediate discontinuation.
Please see full Prescribing Information.